Securing Medical Devices
Protect and monitor devices, and comply with industry standards
Medical devices merge the physical and IT worlds.
Product security standards such as the FDA Guidance for medical/healthcare software, NISTIR 8259, UL2900, and US Executive Order 14028 require medical-device manufacturers (MDMs) to protect their medical devices against cyberattacks. MDMs should harden their connected devices and assure supply-chain security, by putting in place a defined set of controls and management processes.
Trusted by
Authenticate all software/firmware files and updates. Protect data files.
XGuard's Allow Lists and Access Control prevent file and data tampering. Via a mechanism which is automatically created and has low performance impact, each needed application or process can be allowed to run broadly. XGuard allows restricting file operations such as remove/rename/read/write, and it also enables defining elements which are to be exempt from specific rules.
See a demo of how to automatically create Application Allow Lists and apply Access Control as part of your Linux distro, or your firmware build environments.
Track and manage attack attempts.
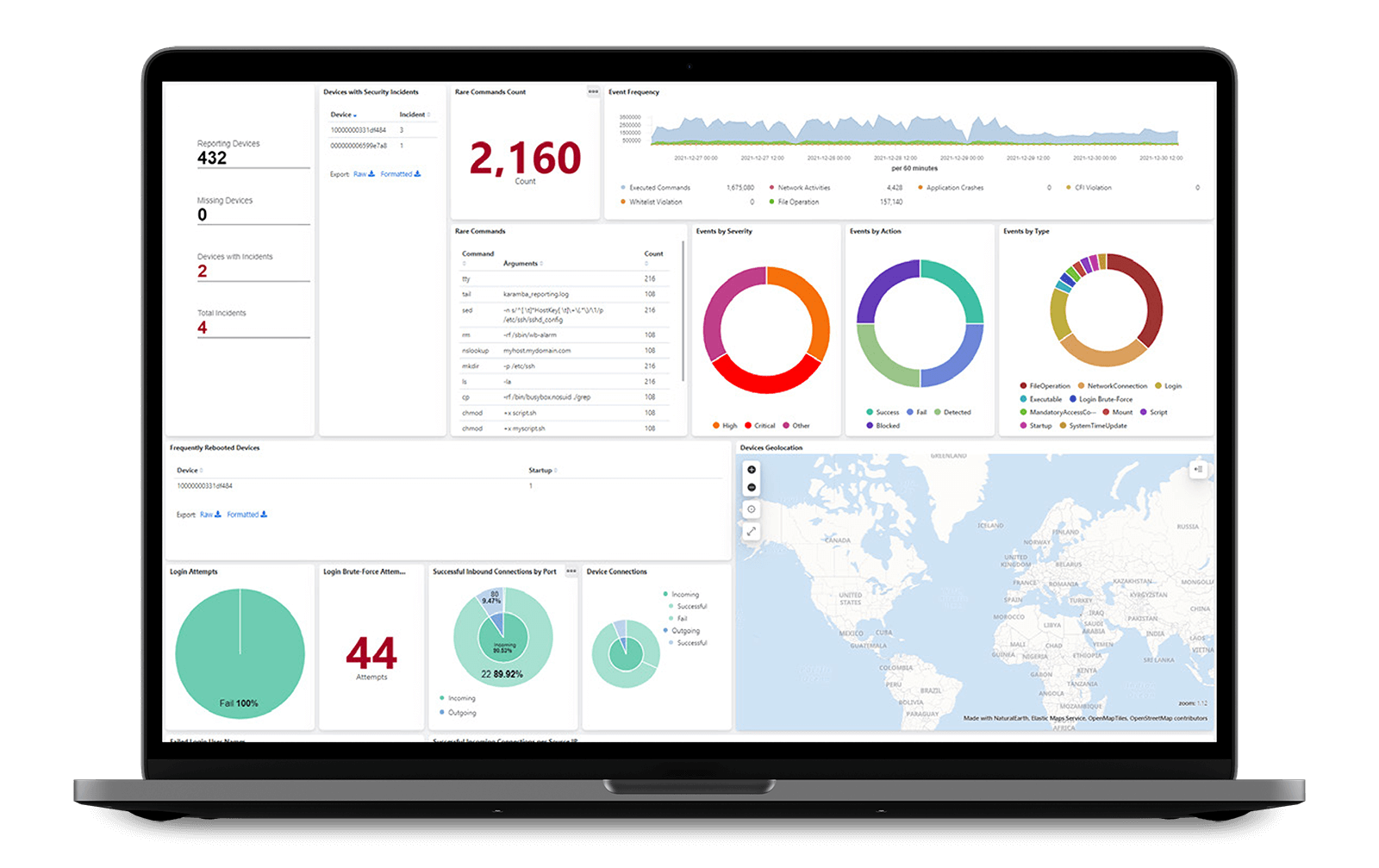
XGuard comes with an initial set of event-reporting templates for various types of security and system events (authentication, connections, commands, crashes, CPU load, file operations and more). Reports can be stored locally and/or sent to a custom server, with negligible performance impact. No time or effort is needed to develop such features as part of the device logic.
See how to automatically set up custom reporting for event detection and logging.
Continuously and automatically manage vulnerabilities and risks.
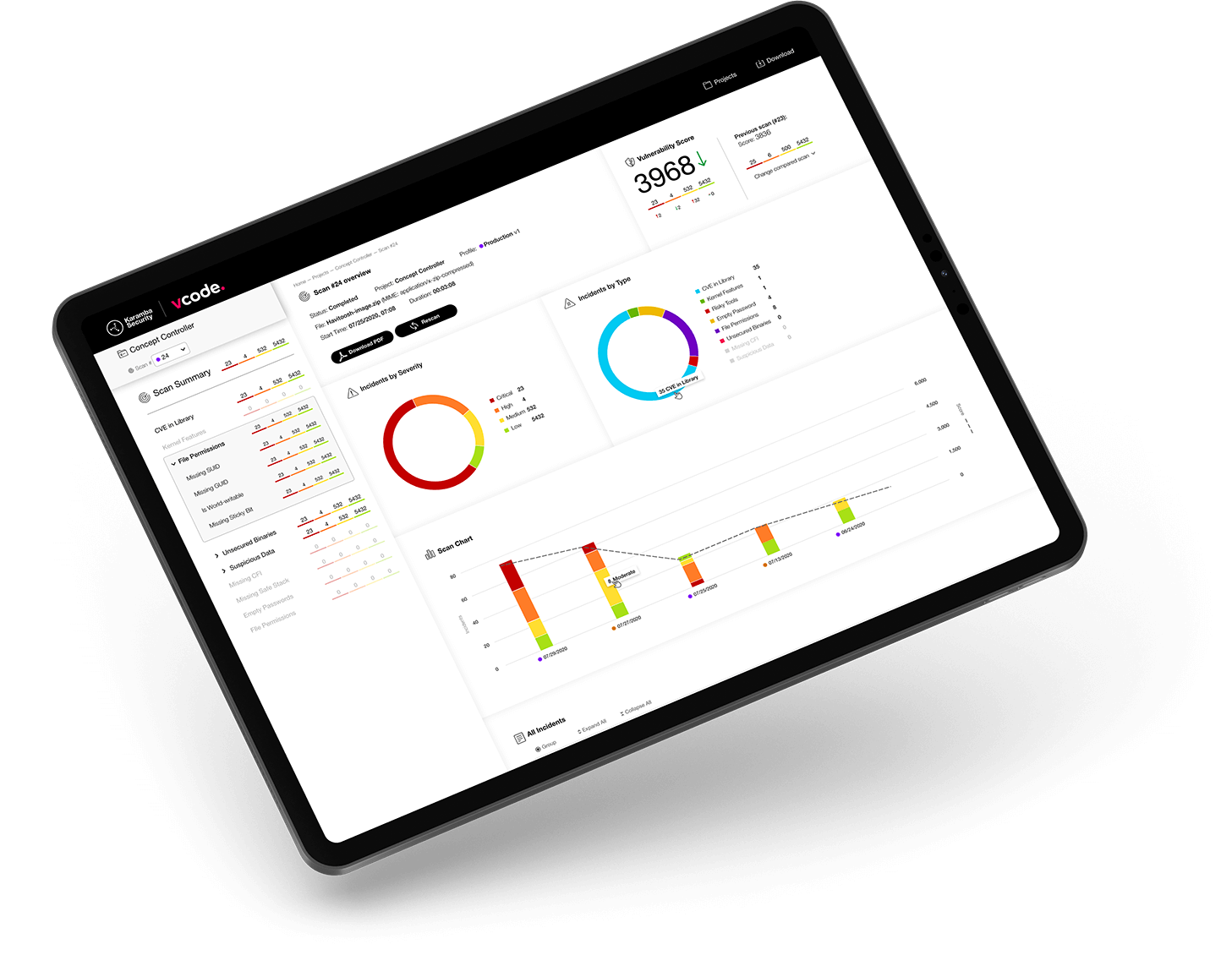
VCode provides automated binary analysis and vulnerability management, across third-party and internally-developed binaries alike. It can be integrated into the CI/CD pipeline, and it is continually updated with new vulnerabilities. VCode identifies the Software Bill of Materials (SBOM), as well as security vulnerabilities, misconfigurations, authentication glitches and risky tools.
Perform adequate security testing.
Karamba’s Penetration Testing services enable device manufacturers to cost-effectively validate their product release prior to production. Embracing the continuous pen-testing approach, the services follow the shift-left practice which recommends starting pen testing early – including at the development stage.
Learn more about Karamba Products for Medical Devices

Medical Device Security Solution Brief
Read about Karamba’s cybersecurity products and how they protect medical devices.

XGuard for Medical Device Security
Download a short summary of XGuard features and benefits.
VCode for Medical Devices
Aligned to Agile SDLC, VCode helps MDMs validate their code continuously.
Israel
24 HaNagar Street
Hod Hasharon
45277-13
Tel: +972 9 88 66 113
USA
41000 Woodward Ave
Building East, Suite 350
Bloomfield Hills, MI 48304
Tel: +1 833 4KARAMBA








